Resources:
MRI information
The U.S. Food and Drug Administration (FDA) has granted the Barostim System an MR Conditional safety label to indicate patients may now receive MRI exams of the head and neck and lower extremities provided specific guidelines are met. If your patient has device model 2102 or 2104 (Barostim NEO or Barostim NEO2) with lead model 1036 (single lead only), they may be eligible. Please consult the Instructions for Use for a complete list of guidelines.
Click here to view the CVRx MRI Instructions For Use Manual
Unlike pacemakers, ICDs, and CRT devices, Barostim does not sense and respond to electrical activity. For this reason, and the presence of a magnetic switch that automatically pauses therapy during an MRI scan, Barostim requires no pre- or post- MRI scan programming (newest models only).
Patient identification
You have several options to determine what type of CVRx device and lead system is implanted in your patient:
Look at the patient’s ID Card
Examine the patient’s medical records
Call CVRx technical support at 1-763-416-2343
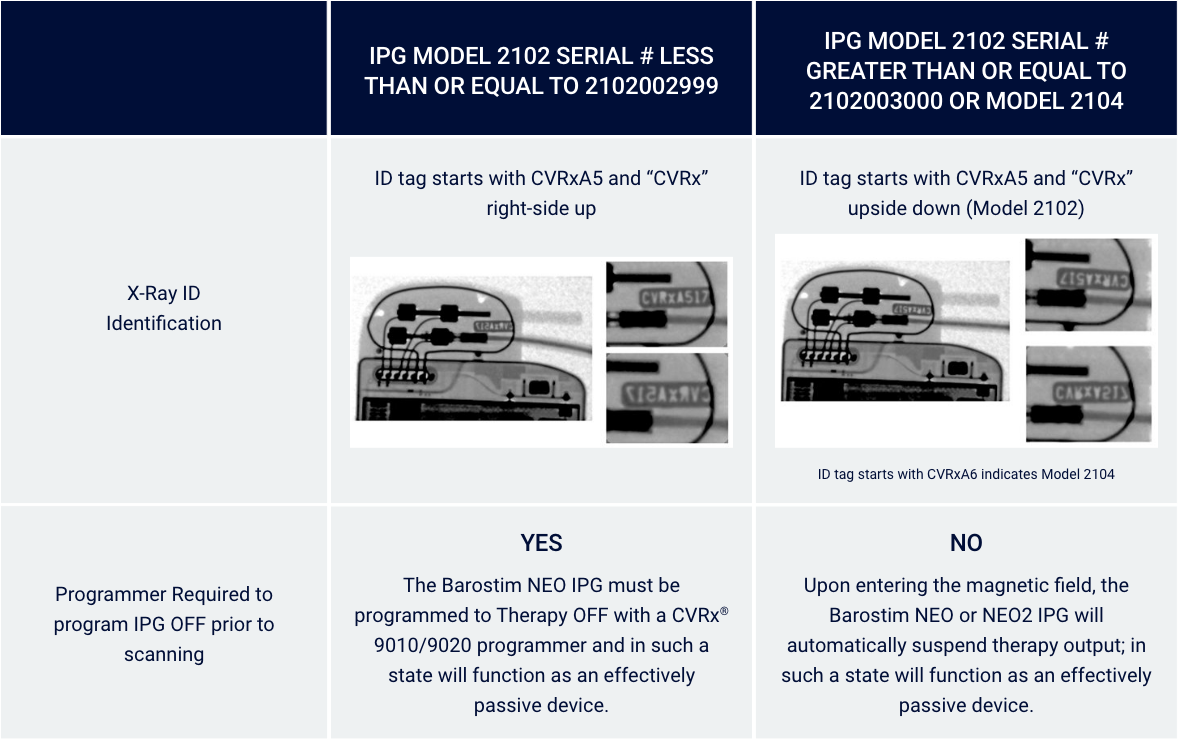
Identify through X-Ray
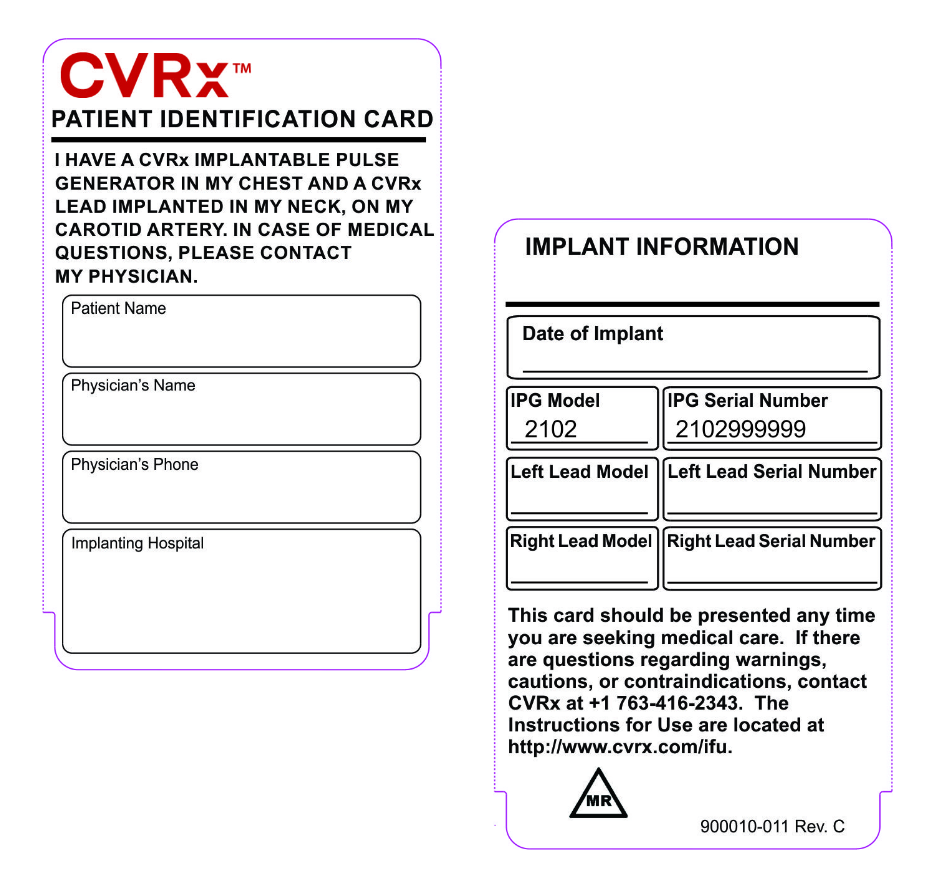
Patient ID card
There is an MR TRIANGLE icon on the back of the Patient ID Card if your patient has the device model 2102 or 2104 (Barostim NEO and Barostim NEO2) with lead model 1036 (single lead only). If so, they are eligible for MRI scans of the head and neck and lower extremities provided specific guidelines in the manual are followed.
Note: For devices implanted prior to the labeling update, the patient ID card will not have the MR Conditional Triangle icon.
If your patient has the device model 2100 (Barostim Legacy) and/or lead models 1010, 1014, or 1037, repaired leads, known damaged leads, or more than one implanted lead, they are not eligible for MRI scans.
However, they are eligible to have CT scans, X-Rays and ultrasound images taken anywhere in the body.
Identify through x-ray